Technologies
AVIRMAX TECHNOLOGIES
AAV Capsid Engineering
Gene therapy (GT) is bound to be the future of biomedicine. Recombinant AAVs (rAAVs) are the most promising vectors because they transduce dividing and quiescent cells, have long-term gene expression in vivo, and low toxicity and inflammation in target tissues. Currently AAV is a popularly used vector in gene therapy clinical trials.
GT AAV vectors contain two parts, the capsid and the gene of interest (GOI). The capsids of AAV vectors are derived either from natural AAV viral isolates or by engineering capsids through rational design, directed evolution, or computer-guided technologies. Capsid quality and target cell tropism are important features of rAAV products to achieve safety and efficacy in gene therapy. This is particularly important for the development of rAAV to deliver a GOI into retinal cells for the treatment of ocular diseases. Our AAV capsid engineering technologies provide an effective solution for a simple administration of rAAV vector via an intravitreal (IVT) route with high efficiency of transduction of retinal cells. Use of minimal capsid formation protein (MCFP) helps us greatly to achieve high efficiency and accuracy of candidate screening for AAV and its receptor binding (Fig. 1).
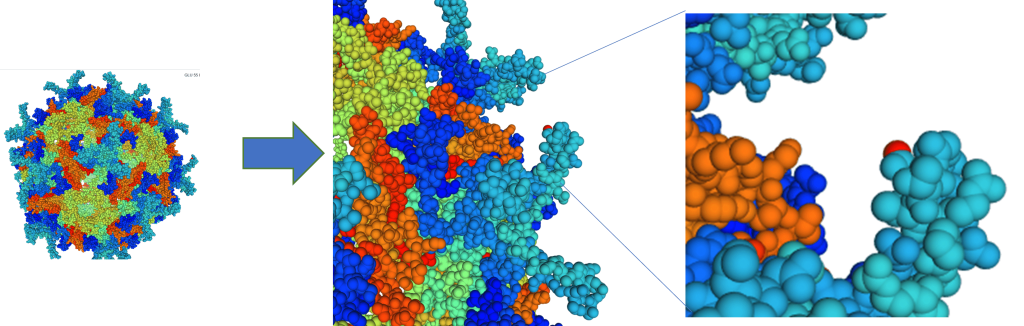
FIG. 1. Engineered AAV2 Capsid Structures
(Capsid structure predicted by computer modeling)
Our directed evolution approach with capsid libraries provides a powerful tool to screen more AAV capsid for various application.
Engineered AAV capsids are selected using rodent and large animal model systems to determine the locality of AAV vectors and cell types transduced in retinal layers. Avirmax’s proprietary capsid delivers GOI targeting retinal cells efficiently via IVT administration. The target gene (green fluorescent protein, GFP) carried by AAV vectors were detected in the ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer nuclear layer (ONL), inner segment (IS), outer segment (OS), and retina pigment epithelium (RPE) layer (Fig. 2).
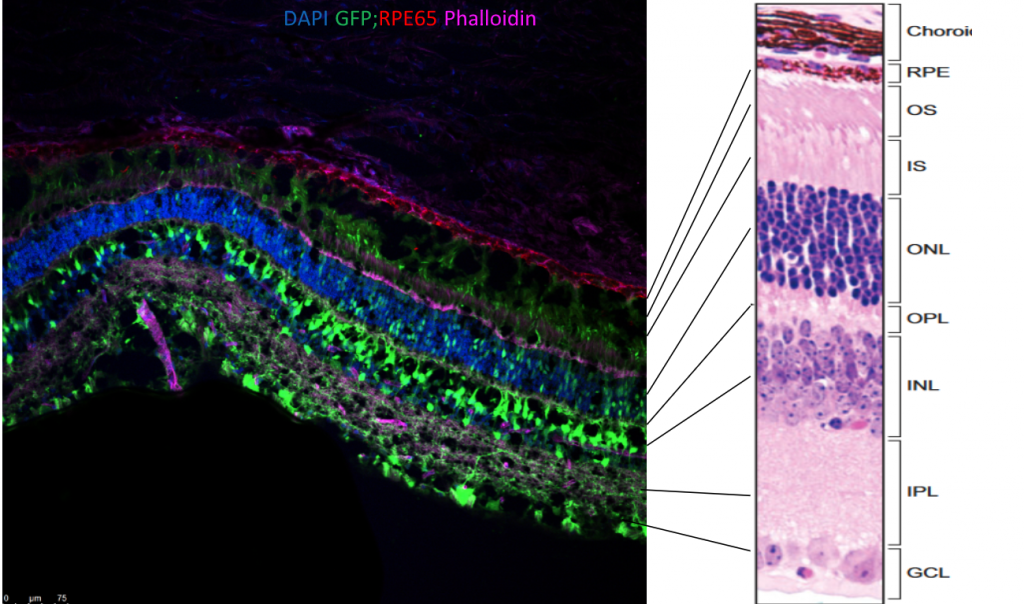
FIG. 2. Confocal image showing retina locality and cell types transduced 25 days prior by AAV2-GFP vector delivered intravitreally

FIG. 3. Fluorescent IHC image showing monkey retina GFP locality and cell types 35 days after AAV2.N54-GFP vector delivered intravitreally
Enhanced Gene Expression Technology
Avirmax’s proprietary transgene optimization technology has demonstrated a significant enhancement of gene expression for its lead GOIs. Below is an example of target gene expression before and after optimization using the Enhanced Gene Expression Technology
A
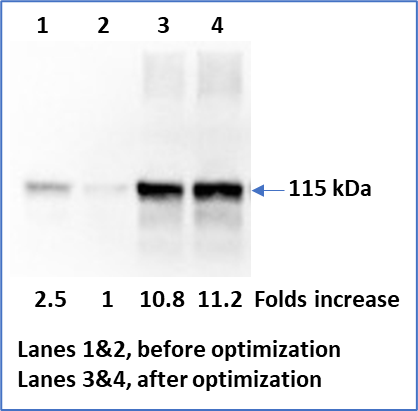
B

FIG. 3. Comparison of Target Gene Expression before and after Optimization Using the Enhanced Gene Expression Technology
Equal amount of plasmid DNA (A) or AAV vectors (B) carrying a target gene was used to transfect/transduce HEK293 cells for 48 hours and media were collected and analyzed with a specific antibody against the target protein. Arrow indicates the molecular weight of the target protein.

